by Axel Norberg and Paul Gatenholm, Chalmers University of Technology, Sweden. November 2019.
Introduction
Alginates are a family of biopolymers which are FDA approved for several indications and have been successfully used in tissue engineering for many years. [1] The reason for their widespread use is due to their cytocompatibility, biocompatibility, water holding capacity with good mechanical properties and ease of cross-linking. These properties are derived from their chemical structures. Alginates are linear polysaccharides composed of two acids: α-L guluronic acid (G) and β-D mannuronic acid (M), see figure 1.
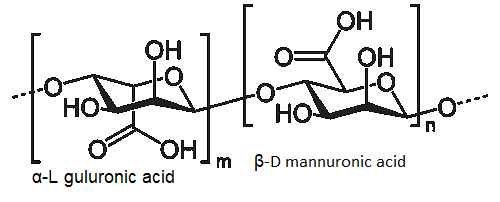
Figure 1. Chemical formula of alginic acid, the two building blocks α-L guluronic acid and β-D mannuronic acid.
The alginate biopolymers are composed of G-, GM- or M-blocks, and how the G and M-blocks are organized affect the properties. The G-blocks increase gel stiffness as they participate in the crosslinking whilst the GM- and M-blocks increase the flexibility of the biopolymer. There are also more subtle effects that arise from the formation of the polymer such as immunogenicity and cell adherence. The molecular weight (MW) also has an effect on crosslinking and the pre-gel solution, where low-MW leads to less stiffness than high-MW for the same crosslinking parameters. [2] High MW also results in a more viscous solution prior to crosslinking, which affect the mixing with cells or proteins. [3] Alginates are crosslinked ionically when they come in contact with divalent cations, such as calcium, barium and strontium.
The level of crosslinking and the stiffness of the alginate post crosslinking can be controlled through the concentration of the crosslinking agent, most commonly calcium chloride (CaCl2), which allows for great tunability of the construct. The crosslinking incurred when alginate is subjected to CaCl2 is however very rapid and not ideal for all applications, other calcium based crosslinkers with slower gelation rates is calcium sulphate (CaSO4) and calcium carbonate (CaCO3). [3]
Alginates have seen a steady increase in research interest, highlighted by the increasing number of publications assessing/utilizing them for tissue engineering purposes as scaffolds, cell delivery and more recently as bioinks. [4] Alginate solutions are shear thinning, which means that viscosity decreases when shear rate is increased and thus can be used with both bioprinting and casting fabrication methods. There are different scaffold fabrication methods available:
Microfluidic Fibre-shaped coaxial is an attractive way of fabricating cell laden (cell containing) constructs. It consists of three main steps: the formation of a core-shell hydrogel, culturing cells within this core, the final step is the degradation of the alginate scaffold which leaves only the cultured cells with produced ECM. This method has been used to create cardiomyocyte-fibres, which show contraction, endothelial -fibres, which form tubular structures with monolayers forming along the direction of the fibre and cortical fibres in which dendrites formed neuronal networks. These cell fibres provide a microenvironment which contribute to regulate cell-cell interactions and functions. [5]
Freeform reversible embedding of hydrogels (FRESH) is a method that allows for complex structures to be formed. The alginate solution is dispensed in a gelatine slurry which supports the alginate prior to crosslinking. This allows for the creation of overhangs and hollow structures, see figure 2 for overview and figure 3 for example of biofabrication of a blood vessel. [6] [7] When the construct is finalized, and the alginate crosslinked, heating the gelatine to 37 degrees Celsius liquefies it allowing for handling of the construct with complex architecture.
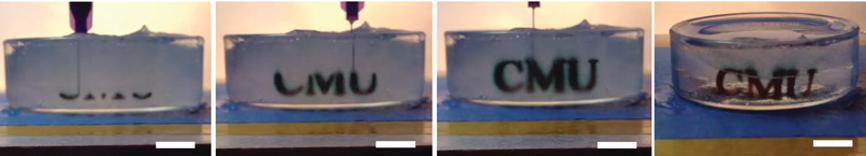
Figure 2. Overview of printing Alginate into a gelatin slurry with melting of the support bath post crosslinking[2]
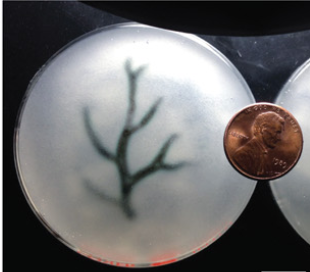
Figure 3. A model based of a MRI of a coronary arterial tree made of alginate is printed as a hollow structure using the FRESH method[2]
Casting tubes and vascular trees
Alginate can of course be casted to create hollow structures as well, in this process a sacrificial object is covered with the alginate. Post crosslinking the sacrificial object is removed and leaves a hollow channel in its wake. This can also be coupled to scans of native tissue, see figure 4. [8] Overall a very versatile method that allows for the formation of complex structures. [7]
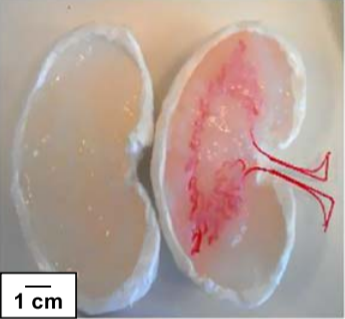
Figure 4. A model made from the CT-scan of a kidney. The sacrificial material can be degraded and provide a hollow channel for perfusion [8]
Spheroid formation
Encapsulation of cells within alginate spheroids is a simple method to produce a stable and uniform 3D-culturing system. These spheroids can be achieved through electrostatic droplet formation, the spheroids are produced by dispensing alginate into an electrostatic field generated between the nozzle and a calcium chloride bath, see fig 5. [9]
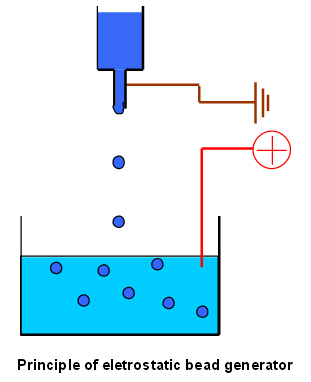
Figure 5. A schematic of the electrostatic droplet setup. The polymers within the syringe is being dispensed and the electrical field drives the droplet into the crosslinking solution
Varying the gauge of the needle, the strength of the electrostatic field, distance between the nozzle and the bath, the concentration of the calcium bath and the crosslinking time allows for tailored spheroid formation. [10] [11]
Modified alginate
The alginate can also be modified with proteins to better support cell growth and function. On example is RGD sites which act as cell attachments sites. These sites allow for a cell environment which promotes normal cell alignment and a good starting ground for the production of the cells own extracellular matrix proteins. [12] There is also the possibility of combining alginate with other compounds to form composites which fit into many different niches. Some of these possible alginate composites include, synthetic polymers, biopolymers, ceramics, proteins, bioglass among others. The most common ones are alginate-polymers/biopolymers blends and composited, alginate-proteins and alginate-ceramics. Common polymer blends and composites include, poly(lactic-co-glycolic acid)(PLGA), Poly(ethylene-glycol)(PEG), cellulose and chitosan. For the alginate-proteins complexes the most common building blocks are collagen and gelatine. Both blends show great potential and have already been used in many different areas of tissue engineering. However, the third composite, alginate-ceramic, has a narrower field of application as it is exclusively used for bone tissue engineering. Here the alginate is commonly combined with hydroxyapatite materials which closely resemble bone ECM. [2] [3] [4]
The alginate solutions can also be mixed to form blends with less refined autologous tissue, such as mechanically processed adipose tissue. This is a very recently discovered method and shows great potential as an alternative to traditional fat grafting. This blend contains a cocktail of stem and progenitor cells, ECM-proteins and possesses great cytocompatibility and material characteristics which provides excellent regenerative properties. [13]
As mentioned previously alginate crosslinks when exposed to divalent cations, and while this is an easy and simple way of crosslinking it does not always produce the best results. As the crosslinking is based on ionic interactions it is a reversible process, which means that the stability of the scaffold is dependent on external factors. The stability of the construct is also dependent on diffusion rates of the ions. These limitations can be overcome through mixing of polymers, for example combining alginate with gelatine methacrylate (GelMA) and a photo initiator allows both ionic and covalent crosslinking. Scaffolds can also be designed in a way that allows for crosslinking solution to penetrate deeper into the constructs, see fig 6. [14]

Figure 6. A schematic overview of crosslinking levels for two constructs. Allowing for crosslinking solution to flow into the scaffold decreases the diffusion lengths for the ions.
Alginate for wound healing
The key function of traditional wound dressings is to act as a barrier, keeping pathogens out whilst allowing for evaporation of wound fluids. More modern dressings utilize a moisturizing layer in order to promote normal wound healing. The Alginate can be lyophilized to form absorbent fabrics that forms the gel when in contact with wound exudate. [15]
Alginate used for bone engineering
When engineering scaffolds for bone regeneration the ideal scaffold is one which provides mechanical strength while maintaining conditions which promote cell adhesion, proliferation and differentiation. Alginate scaffolds alone cannot support the mechanical loads required for bone tissue engineering, therefore there are combined with inorganic materials such as hydroxyapatite which provides strength and promote new bone formation. It has also been shown that alginate and calcium composites can be treated to achieve high levels of porosity, over 80%, which also work towards promoting natural cell proliferation. [4] [16]
Alginate for in vitro models
In addition to the previously mentioned applications which are focused on treating human injuries, alginates have been successfully applied as scaffolds for the development of in vitro disease models. Many of the models developed focus on cancer metabolism and are often combined with spheroid formation. It has been shown that alginate-based hydrogels allow for co-cultures of cancer cell lines with high cell viability in vitro for over 30 days. [9] [17] [18]
References
[1] Sun, Jinchen, and Huaping Tan. “Alginate-Based Biomaterials for Regenerative Medicine Applications.” Materials (Basel, Switzerland), vol. 6, no. 4, 2013, pp. 1285–1309, DOI: 10.3390/ma6041285 http://www.ncbi.nlm.nih.gov/pubmed/28809210
[2] Freeman, Fiona E., and Daniel J. Kelly. “Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues.” Scientific Reports, vol. 7, no. 1, Dec. 2017, DOI: 10.1038/s41598-017-17286-1.http://www.nature.com/articles/s41598-017-17286-1
[3] Lee, Kuen Yong, and David J Mooney. “Alginate: properties and biomedical applications.” Progress in polymer science, vol. 37, no. 1, Jan 2012, pp. 106-126. DOI:10.1016/j.progpolymsci.2011.06.003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3223967/
[4] Venkatesan, Jayachandran et al. “Alginate composites for bone tissue engineering: A review” International journal of biological macromolecules, vol. 72, Jan. 2015, pp 269-281. DOI: 10.1016/j.ijbiomac.2014.07.008 https://www.sciencedirect.com/science/article/pii/S0141813014004735
[5] Onoe, Hiroaki et al. “Metre-long cell-laden microfibres exhibit tissue morphologies and functions” Nature Materials, vol. 12, Mar 2013, pp. 584–590, DOI:10.1038/nmat3606 https://www.nature.com/articles/nmat3606
[6] Hinton, Thomas J., et al. “Three-Dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels.” Science Advances, vol. 1, no. 9, Oct. 2015, p. e1500758, DOI: 10.1126/sciadv.1500758. http://advances.sciencemag.org/content/1/9/e1500758.full
[7] Justin, Alexander W., et al. “Multi-Casting Approach for Vascular Networks in Cellularized Hydrogels.” Journal of The Royal Society Interface, vol. 13, no. 125, Dec. 2016, p. 20160768, DOI: 10.1098/rsif.2016.0768. https://royalsocietypublishing.org/doi/10.1098/rsif.2016.0768
[8] Sämfors, Sanna, et al. “Biofabrication of Bacterial Nanocellulose Scaffolds with Complex Vascular Structure.” Biofabrication, vol. 11, no. 4, 2019, p. 045010, DOI: 10.1088/1758-5090/ab2b4f. http://www.ncbi.nlm.nih.gov/pubmed/31220812
[9] Akeda, Koji et al. “Three-dimensional alginate spheroid culture system of murine osteosarcoma”. Oncology Reports, vol. 22, no. 5, Nov 2009, pp. 997-1003. DOI: 10.3892/or_00000527 https://www.spandidos-publications.com/or/22/5/997
[10] Salg, Gabriel Alexander, et al. “The Emerging Field of Pancreatic Tissue Engineering: A Systematic Review and Evidence Map of Scaffold Materials and Scaffolding Techniques for Insulin-Secreting Cells.” Journal of Tissue Engineering, Jan. 2019, DOI:10.1177/2041731419884708 https://journals.sagepub.com/doi/full/10.1177/2041731419884708#articleCitationDownloadContainer
[11] Burgarski, Branko et al. “Electrostatic droplet generation: Mechanism of polymer droplet formation.” AICHE Journal, vol. 40, no. 6, Jun. 1994, pp. 1026-1031. DOI: 10.1002/aic.690400613 https://aiche.onlinelibrary.wiley.com/doi/abs/10.1002/aic.690400613
[12] Shachar, Michal, et al. “The Effect of Immobilized RGD Peptide in Alginate Scaffolds on Cardiac Tissue Engineering.” Acta Biomaterialia, vol. 7, no. 1, 2011, pp. 152–62, DOI: 10.1016/j.actbio.2010.07.034. http://www.ncbi.nlm.nih.gov/pubmed/20688198,
[13] Säljö, Karin, et al. “Successful Engraftment, Vascularization, and In Vivo Survival of 3D-Bioprinted Human Lipoaspirate-Derived Adipose Tissue.” Bioprinting, vol. 17, Mar. 2020, DOI: 10.1016/j.bprint.2019.e00065,. https://www.ncbi.nlm.nih.gov/pubmed/31220812
[14] Pepelanova, Iliyana, et al. “Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting.” Bioengineering, vol. 5, no. 3, 18 July 2018, p. 55, DOI: 10.3390/bioengineering5030055. https://www.ncbi.nlm.nih.gov/pubmed/30022000
[15] Barnett, S E, and S J Varley. “The Effects of Calcium Alginate on Wound Healing.” Annals of the Royal College of Surgeons of England, vol. 69, no. 4, 1987, pp. 153–5,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2498465/
[16] Fuji, Takeshi et al. “Octacalcium Phosphate–Precipitated Alginate Scaffold for Bone Regeneration” Tissue Engineering Part A, vol. 15, no. 11, Sep 2009, pp. 3525-3535, DOI: 10.1089/ten.tea.2009.0048. https://www.liebertpub.com/doi/10.1089/ten.tea.2009.0048
[17] Jiang, Tao et al. “Bioprintable Alginate/Gelatin Hydrogel 3D In Vitro Model Systems Induce Cell Spheroid Formation.” Journal of Visualized Experiments (137), e57826, Jul. 2018, DOI:10.3791/57826 https://www.ncbi.nlm.nih.gov/pubmed/30010644
[18] Cui, Xiaolin et al. “Advances in multicellular spheroids formation.” Journal of The Royal Society Interface, vol. 14, no.127, pii: 20160877, Feb 2017. DOI: 10.1098/rsif.2016.0877. https://royalsocietypublishing.org/doi/10.1098/rsif.2016.0877
