- Viscosity [mPa*s] : < 20
- Appr. Mw [kDa] : < 75
- G/M Ratio : ≤ 1
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : ≤ 100
PRONOVA® UltraPure or Sterile Sodium Alginates

NovaMatrix® is the world’s leading producer of medical grade Sodium Alginates: we have been manufacturing UltraPure Alginates since 1988. PRONOVA® UltraPure and PRONOVA® Sterile sodium alginates are our flagship products.
PRONOVA® alginates, a solid track record of safety and efficacy –
PRONOVA® alginates are very versatile, well-characterized, safe and bio-compatible marine biopolymers. They have been purified for biomedical and pharmaceutical applications. PRONOVA® UltraPure Alginates have been used in commercial medical applications for over two decades.

Chemistry
Alginate is a naturally-occurring biopolymer extracted from brown seaweed.
In contrast to most other polysaccharides, Alginates can form hydrogels at constant temperature, in presence of divalent cations such as Ca2+, Ba2+ or Sr2+. This unique property is particularly useful in applications involving fragile materials like cells or tissue with low tolerance for higher temperatures. For more information, please click on the hyperlink PRONOVA® Alginates – Hydrogels formation and fill in a simple contact form.
Properties
- Form 3-dimentional hydrogel matrixes in presence of divalent cations
• Ability to form beads to entrap cells and therapeutic actives
• Ability to form 3D scaffolds, foams and other structures - Cell affinity
• Cell entrapment in alginate matrixes
• Controlled viscosity and gelation based on alginate type and cation selection Wide range of viscosities and gel strengths available - Cold / hot water solubility
- Rheology modification
- Suspending agent
- Film forming
- Naturally-occurring biopolymer with 20+ years in commercial applications
• Safe, biocompatible - Ultrapure, for medical use
- Long shelf-life: from 3 to 5 years, depending on the product

Safety and toxicology
The safety and toxicology profile of PRONOVA® UltraPure sodium alginate is described in a Drug Master File (DMF) submitted to the US FDA.
Applications

- Encapsulation of living cells and therapeutic proteins for cell therapy and advanced drug delivery
- Bone putty binder for tissue engineering and reconstruction
- Cryoprotection for cell and tissue
- 3D bioprinting
- Foams and cell tissue scaffolds
- Cell culture
- Hydrogels
- Advanced drug delivery
- Implants
- Anti-adhesion
- Wound management
- Medical device coating, etc.
Product selection
PRONOVA® UltraPure Alginates – PRONOVA® UP Product Table
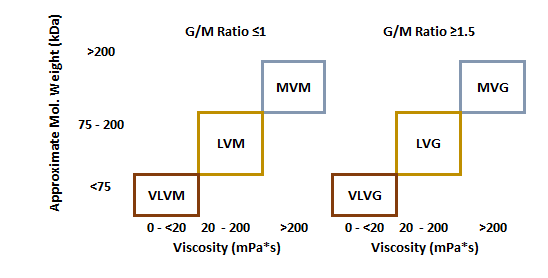
All our PRONOVA® UltraPure Alginates have ≤ 100 EU / g endotoxins specifications as well as ≤ 100 cfu /g total viable count specifications.
We have the ability to supply PRONOVA® UltraPure Alginates at lower endotoxins specifications, as well as tailor-made grades. Please connect with us for more information.
The shelf-life of PRONOVA® UltraPure Alginates varies by grade. If you would like to learn more about PRONOVA® Alginates product shelf-life, please click on the hyperlink and fill in a simple contact form.
PRONOVA® Sterile Alginates – PRONOVA® S Product Table
All our PRONOVA® Sterile Alginates have ≤ 100 EU / g endotoxins specifications.
We have the ability to supply PRONOVA® Sterile Alginates at lower endotoxins specifications, as well as tailor-made grades. Please connect with us for more information.
The shelf-life of PRONOVA® Sterile Alginates varies by grade. If you would like to learn more about PRONOVA® Alginates product shelf-life, please click on the hyperlink and fill in a simple contact form.
Alginate is a linear polysaccharide consisting of (1,4)-linked b-D-mannuronate (M) and its C-5 epimer α-L-guluronate (G).
The monomers can appear in homopolymeric blocks of consecutive G-residues (G-blocks), consecutive M-residues (M-blocks), alternating M and G-residues (MG-blocks) or randomly organized blocks.
PRONOVA UP VLVM
PRONOVA UP LVM
- Viscosity [mPa*s] : 20-200
- Appr. Mw [kDa] : 75-200
- G/M Ratio : ≤ 1
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : ≤ 100
PRONOVA UP MVM
- Viscosity [mPa*s] > 200
- Appr. Mw [kDa] : > 200
- G/M Ratio : ≤ 1
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : ≤ 100
PRONOVA UP VLVG
- Viscosity [mPa*s] : < 20
- Appr. Mw [kDa] : < 75
- G/M Ratio : ≥ 1.5
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : ≤ 100
PRONOVA UP LVG
- Viscosity [mPa*s] : 20-200
- Appr. Mw [kDa] : 75-200
- G/M Ratio : ≥ 1.5
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : ≤ 100
PRONOVA UP MVG
- Viscosity [mPa*s] : > 200
- Appr. Mw [kDa] : > 200
- G/M Ratio : ≥ 1.5
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : ≤ 100
Product
Viscosity [mPa*s]
Appr. Mw [kDa]
G/M Ratio
Endotoxins [EU/g]
Total viable count [cfu/g]
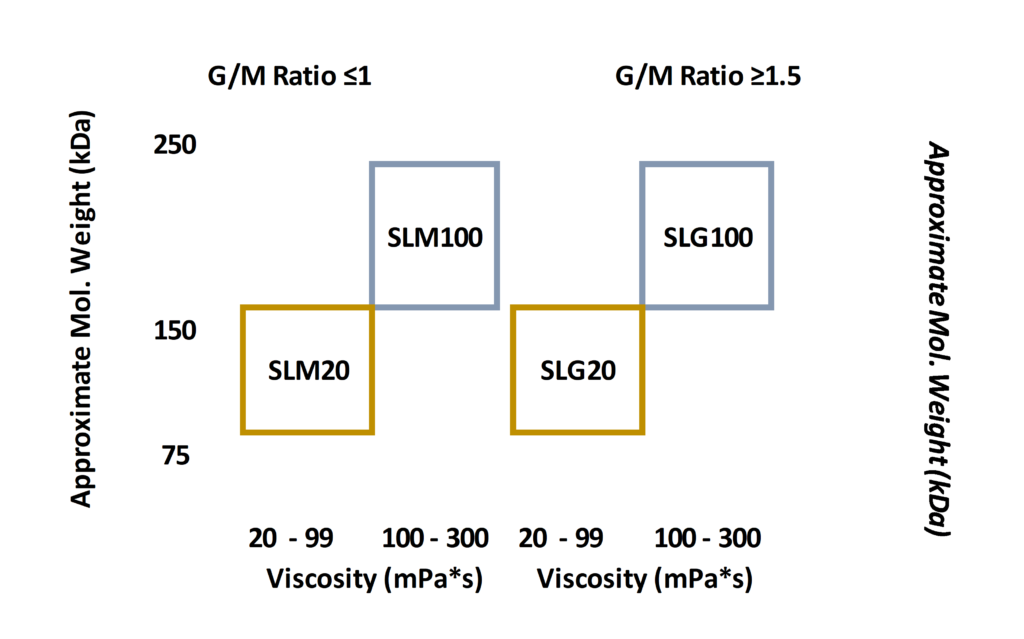
PRONOVA SLM20
- Viscosity [mPa*s] : 20-99
- Appr. Mw [kDa] : 75-150
- G/M Ratio : ≤ 1
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : Sterile
PRONOVA SLM100
- Viscosity [mPa*s] : 100-300
- Appr. Mw [kDa] : 150-250
- G/M Ratio : ≤ 1
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : Sterile
PRONOVA SLG20
- Viscosity [mPa*s] : 20-99
- Appr. Mw [kDa] : 75-150
- G/M Ratio : ≥ 1.5
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : Sterile
PRONOVA SLG100
- Viscosity [mPa*s] : 100-300
- Appr. Mw [kDa] : 150-250
- G/M Ratio : ≥ 1
- Endotoxins [EU/g] : ≤ 100
- Total viable count [cfu/g] : Sterile
Product
Viscosity [mPa*s]
Appr. Mw [kDa]
G/M Ratio
Endotoxins [EU/g]
Total viable count [cfu/g]
NOVATACH MVG GRGDSP (GRGDSP-coupled high G-content high Mw alginate)
- G/M Ratio : ≥ 1.5
NOVATACH VLVG 4GRGDSP (GRGDSP-coupled high G-content low Mw alginate)
- G/M Ratio : ≥ 1.5
NOVATACH LVM GRGDSP (GRGDSP-coupled alginate)
- G/M Ratio : ≤ 1
NOVATACH G VAPG (VAPG-coupled alginate)
- G/M Ratio : ≥ 1.5
NOVATACH M REDV (REDV-coupled alginate)
- G/M Ratio : ≤ 1

